Pamela Garzone
Pfizer Inc., USA
Title: An engineered pH sensitive PCSK9 antibody to enhance pharmacokinetic and pharmacodynamic properties
Biography
Biography: Pamela Garzone
Abstract
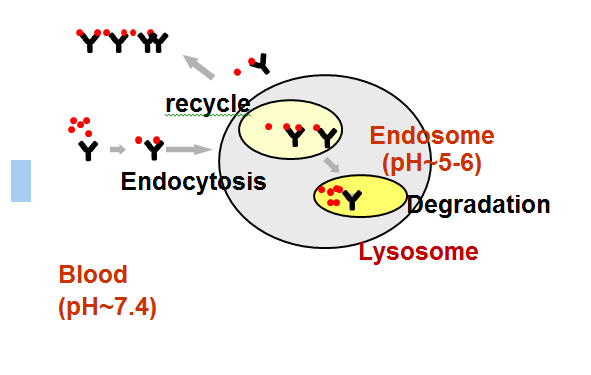
Introduction & Aim: Proprotein Convertase Subtilisin/Kexin Type 9 Inhibitor (PCSK9) binds to and down regulates low-density lipoprotein receptor (LDL-R) levels on hepatocytes, resulting in LDL-cholesterol (LDL-C) lowering. A PCSK9 monoclonal antibody (mAb) was engineered with pH-sensitive binding to enhance pharmacokinetic (PK) and pharmacodynamics (PD) properties.
Methodology: In men and women (18–70 years) with hypercholesterolemia and prescribed statins, single 0.3, 1, 3, or 6 mg/kg subcutaneous (SC) RN317 doses or 1, 3, and 6 mg/kg intravenous (IV) were evaluated. Three doses of 300 mg SC every 28 days (Q28d) were also evaluated in another cohort. Key exclusion criteria included poorly controlled diabetes and hypertension.
Results: In humans, absorption was slow and varied, reaching the maximum concentration (Cmax) between 3 and 10.5 days after RN317 SC. The half-life was long (~19–21 days). Absolute bioavailability ranged from 38.5% to 67.5%. Accumulation with repeated dosing was minimal (Rac=1.23). The most frequent adverse events (AEs) were upper respiratory tract infection (n=5), headache (n=4), and diarrhea (n=4). Mean percentage decreases from baseline in LDL-C ranged from 0.2% to 52.5% across the RN317 dosages compared with 5.9% to 15.3% with placebo and maintained to day 29. The magnitude and duration of LDL-C reduction with IV RN317 was generally greater than that observed after SC. Following 3.0 mg Q28d, minimal fluctuation in LDL-C lowering (trough-to-nadir ratio>0.9) was noted throughout the study period.
Conclusions: A long terminal half-life and sustained LDL-C lowering was observed; the mean % LDL-C reduction observed was not as marked as that with other anti-PCSK9 mAbs following single or multiple doses. RN317 was well-tolerated and consistent in safety profile with other anti-PCSK9 mAbs, suggesting the tolerability profile of the drug was not altered with the engineered changes.