
Kobbi Zina
University of Monastir, Tunisia
Title: Comparative repeated toxicity study in rats: Enoxaparin biosimilar product versus reference product
Biography
Biography: Kobbi Zina
Abstract
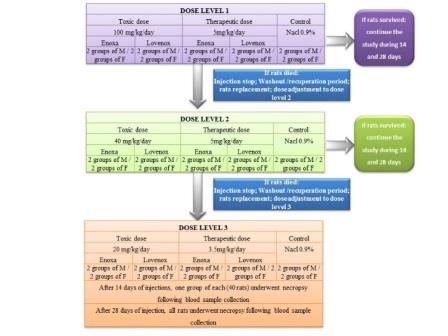
During last decades we observed explosion of biosimilars, which require biosimilarity study including comparative safety evaluation conducted on animals. Enoxaparin is a low molecular weight heparin widely used for the prevention and treatment of thromboembolism and is considered as a biological product. With the development of several enoxaparin biosimilars, real medical concerns about their safety and efficacy were raised. This repeated dose toxicity part of the biosimilarity study consists of preclinical toxicological evaluation of a similar biological version of enoxaparin drug product “Enoxa” manufactured by “Les Laboratories Médis” (Tunisia), compared to the enoxaparin reference drug product “Lovenox” manufactured by Sanofi-Aventis (France). Eighty (80) white Wistar rats were treated with enoxaparin biosimilar, versus the reference product, using subcutaneous therapeutic dose and toxic doses, varying from 3.5 to 100 mg/kg/day. Dose levels were adjusted and ultimately fixed at 3.5 mg/kg/day for a therapeutic dose and 20 mg/kg/day for a toxic dose. A 0.9% sodium chloride solution was used for the control group and the comparative study was conducted over a period of 14 days and 28 days. Animals were observed before and during study, all animal were euthanized at the end of the study design then necropsy, organs sampling and anatomo-histopathology were then performed. Hematology and biochemistry evaluation of relevant parameters was performed on all animals. Comparable effects were observed at all doses and all products with few adverse effects observed at doses 20 mg/kg/day for both enoxaparin biosimilar and reference products. Mortality started at a dose of 40 mg/kg/day and reached 25%, at 100 mg/kg/day for both products. Since results from the similar biological version of enoxaparin drug product “Enoxa” and reference drug product “Lovenox”, have comparable toxicity profile in rats, continuing investigation of biosimilarity on humans to confirm safety and efficacy is suggested.
Recent Publications
1. Kobbi Z, Hfaiedh N, Fenina N (2015) Biosimilar evaluation and structural characterization: A comparison study for enoxaparin. American Journal of PharmTech Research 5(3): 593-607.
Speaker Presentations
Speaker PPTs Click Here





