Day 2 :
Keynote Forum
James Dahlgren and Patrick Talbott
James Dahlgren Medical, USA
Keynote: Cancer incidence in coal combustion waste landfill workers
Time : 09:30-10:15

Biography:
James Dahlgren MD is a Board Certified Internist Retired Assistant Professor from UCLA School of Medicine. He has been in private practice of internal medicine with a sub specialty in toxicology for over 40 years. He has studied and treated thousands of patients with toxic chemical injuries including numerous victims of toxic chemical poisoning. Since 1970’s he has been treating and evaluating people with exposures to toxic chemicals.
Abstract:
Coal-fired power plants in the US are one of the largest toxic polluters in the country. Producing electricity from coal not only releases pollutants into the surrounding environment, it also produces wastes that are typically disposed of at a nearby landfill or settling pond. Studies show both the characteristics and ingredients in this toxic waste and how the individualistic ingredients act on the body, specifically as cancer initiators and promoters. The objective of our research was to examine a population of landfill workers (and family) while reviewing the relevant literature on exposure to coal waste and its ingredients. The results indicate an extraordinary amount of cancers in a small population and in accordance with the literature, it can be concluded that the coal waste exposure significantly increases the risk for developing cancer.
- Exhibitor Hosted Session
Location: Sunset 2
- Pharmacology | Food Toxicology | Reproductive & Developmental Toxicology
Location: Sunset 2

Chair
Anna Radominska-Pandya
University of Arkansas for Medical Sciences, USA

Co-Chair
Diana A Stavreva
National Institutes of Health, USA
Session Introduction
Pamela Garzone
Pfizer Inc., USA
Title: An engineered pH sensitive PCSK9 antibody to enhance pharmacokinetic and pharmacodynamic properties
Time : 12:30-13:00

Biography:
Pamela Garzone has 5 years teaching and research experience at the University of Pittsburgh. She has worked in the biotech-pharma industry in Preclinical and Clinical positions, having increasing responsibilities over this time. She has extensive drug development experience in therapeutic areas such as oncology, hematology, immunology, neuroscience, and cardiovascular and infectious diseases. She joined Pfizer in 2009 as an Executive Director and became the Clinical Team Lead for the Early Development Programs at Rinat, a research unit of Pfizer. In 2015, she was promoted to VP, Group Asset Team Lead, responsible for the development strategy of assets within the Oncology Research Unit. In addition, she is currently the Interim Head of the Early Oncology Development and Clinical Research Group.
Abstract:
Introduction & Aim: Proprotein Convertase Subtilisin/Kexin Type 9 Inhibitor (PCSK9) binds to and down regulates low-density lipoprotein receptor (LDL-R) levels on hepatocytes, resulting in LDL-cholesterol (LDL-C) lowering. A PCSK9 monoclonal antibody (mAb) was engineered with pH-sensitive binding to enhance pharmacokinetic (PK) and pharmacodynamics (PD) properties.
Methodology: In men and women (18–70 years) with hypercholesterolemia and prescribed statins, single 0.3, 1, 3, or 6 mg/kg subcutaneous (SC) RN317 doses or 1, 3, and 6 mg/kg intravenous (IV) were evaluated. Three doses of 300 mg SC every 28 days (Q28d) were also evaluated in another cohort. Key exclusion criteria included poorly controlled diabetes and hypertension.
Results: In humans, absorption was slow and varied, reaching the maximum concentration (Cmax) between 3 and 10.5 days after RN317 SC. The half-life was long (~19–21 days). Absolute bioavailability ranged from 38.5% to 67.5%. Accumulation with repeated dosing was minimal (Rac=1.23). The most frequent adverse events (AEs) were upper respiratory tract infection (n=5), headache (n=4), and diarrhea (n=4). Mean percentage decreases from baseline in LDL-C ranged from 0.2% to 52.5% across the RN317 dosages compared with 5.9% to 15.3% with placebo and maintained to day 29. The magnitude and duration of LDL-C reduction with IV RN317 was generally greater than that observed after SC. Following 3.0 mg Q28d, minimal fluctuation in LDL-C lowering (trough-to-nadir ratio>0.9) was noted throughout the study period.
Conclusions: A long terminal half-life and sustained LDL-C lowering was observed; the mean % LDL-C reduction observed was not as marked as that with other anti-PCSK9 mAbs following single or multiple doses. RN317 was well-tolerated and consistent in safety profile with other anti-PCSK9 mAbs, suggesting the tolerability profile of the drug was not altered with the engineered changes.
Catherine Bennetau-Pelissero
University of Bordeaux, France
Title: Estrogenic isoflavones in modern soy-food and their consequences
Time : 13:00-13:30

Biography:
Catherine Bennetau-Pelissero is a Professor from Bordeaux University. She showed the endocrine disruption properties of soy in fish (trout and Siberian sturgeon) in the 1990s. She explained the difference of sensitivity existing between the two species by a difference in gut and liver metabolism allowing a better bioavailability of isoflavones in sturgeon. She published a screening test for estrogens retained by the OECD. She developed highly specific and sensitive immunological assays for phytoestrogens to assess both their dietary concentrations and their bioavailability in animal and in human biological fluids. She was recognized as an Expert of the Phytoestrogen Properties by the French Safety Authorities since 1996.
Abstract:
Statement of the Problem: Isoflavones are estrogenic compounds whose properties were shown to be deleterious to animal reproduction ever since the 1940’s. There effects were confirmed on reproduction, mammary and pituitary adenocarcinomas by the USA National Toxicology Program in 2008. Since then, several studies showed concern about human and breeding species reproduction and about women established breast cancers. The purpose of this study is to evaluate the beneficial effects of glycosylated estrogenic isoflavones present in large concentrations in soybean and its modern derivatives.
Methodology & Theoretical Orientation: Traditional Asian recipes used to prepare natto, miso, tempeh or tofu traditionally involved prolonged cooking of soybeans in water allowing isoflavones to leak into water. The preparation of these solid foodstuffs implied the elimination of water and therefore of glycosylated isoflavones. These prolonged cooking steps were tested on several soybean preparations and showed a significant removal of isoflavones up to 80%.
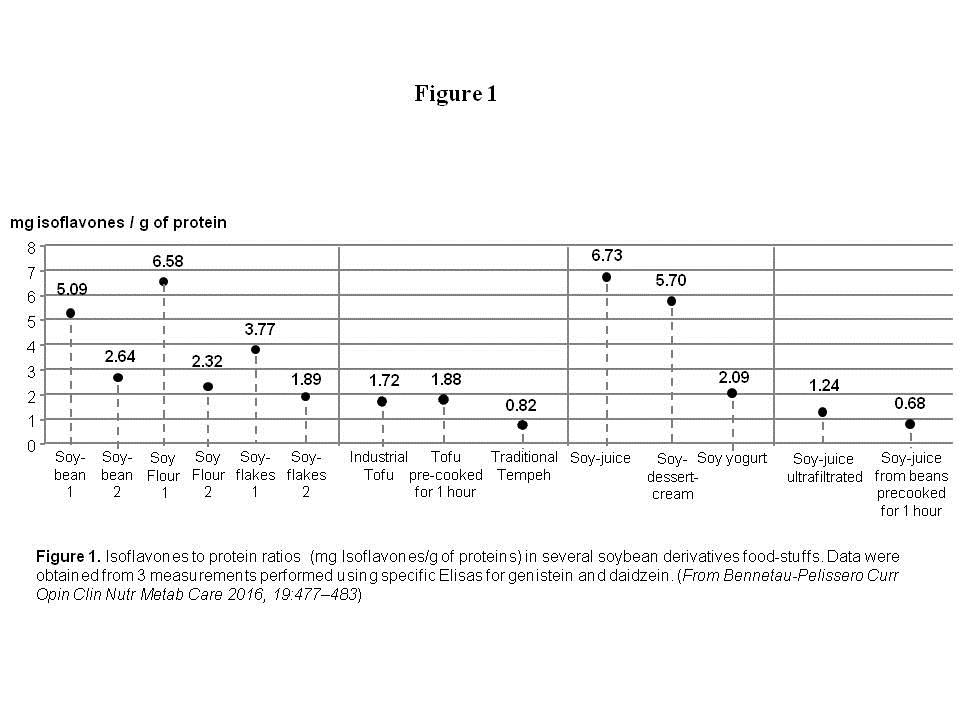
Findings: The isoflavone to protein ratio (mg Isoflavones/g protein) was shown to be higher in modern soy food than in traditional soy-food either prepared following modern techniques or traditional recipes. Soybeans ratio were found to be between 5.1 and 2.7. Soy juice ratios were between 7.4 and 6.7 when tempeh prepared according to a traditional recipe was 0.82. Soy juice prepared from soybeans precooked for 1 hour in water was 0.68.
Conclusion & Significance: The estrogenic isoflavone exposure through modern soy-food, as assessed since the development of analytical methods (1980’s), is most probably higher than the ancestral exposure. This occurs in an environment largely contaminated with other endocrine disrupters with which isoflavones can act synergistically. Because recent studies showed both beneficial and deleterious effects of soy isoflavones on human health, it would probably be better to restrain isoflavones for specific beneficial uses reducing them in the general population and in animal diet.
Recent Publications
1. Bennetau-Pelissero C (2017) Positive or negative effects of isoflavones: Toward the end of a controversy: Response to the letter from Dr Messina and Dr Badger following the publication of the paper by Fernandez-Lopez A, Lamothe V, Delample M, Denayrolles M and Bennetau-Pelissero C. entitled: Removing isoflavones from modern soyfood: why and how? Food Chem. 225: 293-301.
2. Bennetau-Pelissero C (2016) Risks and benefits of phytoestrogens: where are we now? Curr. Opin. Clin. Nutr. Metab. Care 19(6): 477-483.
3. Fernandez-Lopez A, Lamothe V, Delample M, Denayrolles M, Bennetau-Pelissero C (2016) Removing isoflavones from modern soyfood: Why and how? Food Chem. 210: 286-294.
4. Al Abed A S, Sellami A, Brayda-Bruno L, Lamothe V, Noguès X, Potier M, Bennetau-Pelissero C, Marighetto A (2016) Estradiol enhances retention but not organization of hippocampus-dependent memory in intact male mice. Psychoneuro. Endocrinology 69: 77-89.
Michał H Wróbel
Institute of Animal Reproduction and Food Research, Polish Academy of Sciences, Poland
Title: Glyphosate and its most popular product (Roundup) affect the secretion of myometrial contractions regulators from bovine ovary and uterus in vitro
Time : 14:30-15:00

Biography:
MichaÅ‚ H Wróbel is currently an Associate Professor at the Institute of Animal Reproduction and Food Research of the Polish Academy of Sciences in Olsztyn (Poland). He earned his PhD in Faculty of Biology from the University of Warmia and Masury in Olsztyn, in 2008. In his thesis work he pioneered the adverse effect of chlorinated xenobiotics (polichlorinated biphenyls and pesticides) on bovine myometrial contractions. After completing his MSc, he worked as an Assistant Professor in the Department of Cattle Physiology and Endocrinology of the Institute of Animal Reproduction and Food Research, PAS. In 2009, he became an Associate Professor in the Department of Physiology and Toxicology of Reproduction of the Institute of Animal Reproduction and Food Research, PAS.
Abstract:
Glyphosate based herbicides are intensively used in modern agriculture. Therefore, the aim of the study was to investigate the effect of the pure glyphosate and its most popular product (Roundup) on hormonal regulation of the force of myometrial contractions. The myometrial, endometrial, granulosa and luteal cells, as well as the strips of myometrium, from non-pregnant cows, were incubated with the herbicides at doses (0.1, 1 or 10 ng/ml) which were closed to their environmental amount. Neither glyphosate nor Roundup affected the viability of studied cells. Glyphosate stimulated the secretion of testosterone (T) and estradiol from granulosa cells, while Roundup inhibited the T secretion. Both compounds decreased the secretion of progesterone while increased oxytocin (OT) secretion from luteal cells. Roundup significantly decreased prostaglandins (PGF2 and PGE2) secretion from endometrial but not myometrial cells. However, neither basal nor OT-stimulated myometrial contractions were affected by both compounds. The data show that the herbicides impaired the secretory function of ovarian and endometrial cells. Admittedly both compounds change the amount of regulators of uterine contractions, but they do not affect the force of myometrial contractions directly. The data clearly show that these herbicides can support the luteolytic process but may only indicate the potential of these compounds to disturb the regulation of smooth muscle motility.
Recent Publications
1. Wróbel M H, MÅ‚ynarczuk J, Kotwica J (2012) The effect of DDT and its metabolite (DDE) on prostaglandin secretion from epithelial cells and on contractions of the smooth muscle of the bovine oviduct in vitro. Toxicology and Applied Pharmacology 259(2): 152-159.
2. Wróbel M H, Bedziechowski P, MÅ‚ynarczuk J, Kotwica J (2014) Impairment of uterine smooth muscle contractions and prostaglandin secretion from cattle myometrium and corpus luteum in vitro is influenced by DDT, DDE and HCH. Environmental Research 132: 54-61.
3. Wrobel M H, Grzeszczyk M, Mlynarczuk J, Kotwica J (2015) The adverse effects of aldrin and dieldrin on both myometrial contractions and the secretory functions of bovine ovaries and uterus in vitro. Toxicology and Applied Pharmacology 285: 23-31.
4. Wrobel M H, Mlynarczuk J (2017) Secretory function of ovarian cells and myometrial contractions in cow are affected by chlorinated insecticides (chlordane, heptachlor and mirex) in vitro. Toxicology and Applied Pharmacology 314: 63-71.
5. Wrobel M H, Mlynarczuk J (2017) The inhibition of myometrial contractions by chlorinated herbicides (atrazine and linuron), and their disruptive effect on the secretory functions of uterine and ovarian cells in cow, in vitro. Pesticide Biochemistry and Physiology DOI: 10.1016/j.pestbp.2017.01.002.
Young C Kim
Seoul National University, South Korea
Title: Protective effect of betaine on paraquat-induced oxidative stress and pulmonary injury in rats
Time : 15:00-15:30

Biography:
Young C Kim is a Professor of Toxicology at Seoul National University, College of Pharmacy, since 1986. He received his MS and PhD from Purdue University and completed his Post-doctoral research at the National Institute of Environmental Health Sciences (NIEHS), NIH, USA. He has published more than 100 papers in reputed journals. He is a recipient of several prestigious international and national awards including the Thieme Most Innovative Original Paper Award at GA, Society for Medicinal Plant and Natural Product Research, and the Korean Teachers’ Award.
Abstract:
Betaine is a methyl donor utilized in remethylation of homocysteine to methionine in the liver. Our earlier studies revealed that betaine administration elevated hepatic methionine and SAM while reducing homocysteine levels via up-regulation of betaine-homocysteine methyltransferase (BHMT), an enzyme mostly localized in the liver. Betaine was shown to be hepatoprotective against liver injury induced by different toxic substances including ethanol, lipopolysaccharide, and dimethyl nitrosamine, which appeared to be associated with its effect on the metabolism of sulfur-containing amino acids that was extensively impaired by the hepatotoxicants. In a recent study, however, we demonstrated that the metabolism of sulfur-containing amino acids in kidney was also altered significantly in rats fed betaine. In that study the change in renal transsulfuration reactions by betaine was suggested to be secondary, attributable to the elevation of blood methionine level due to the alterations in sulfur-containing amino acid metabolism in the liver. It was therefore, of interest to define the change in the metabolism of sulfur-containing substances by betaine in extra hepatic sites and its pharmacological/toxicological significance. Rats received betaine (1% in drinking water) for 2 weeks prior to intra-tracheal instillation of paraquat (PQ; 0.3 mg/kg). In 2 weeks after PQ instillation, 4-hydroxyproline levels in the lung and oxidative DNA damage were increased significantly, which was effectively prevented by betaine supplementation. Similar results were shown in histopathological assessment of lung tissues. Betaine intake elevated methionine, S-adenosylmethionine (SAM), putrescine and spermidine levels in the lungs significantly. On day 4 after PQ instillation, glutathione (GSH) and its metabolic substrates, including methionine, SAM and cysteine, were all elevated in the lung by betaine supplementation. Elevation of proinflammatory cytokines was also inhibited significantly. It is suggested that betaine may protect the lung from PQ-induced oxidative stress and pulmonary fibrosis most probably via enhancement of antioxidant capacity and polyamine synthesis.
- Genetic Toxicology | Nanotoxicology | Regulatory Toxicology
Location: Sunset 2

Chair
Christopher Busby
Latvian Academy of Sciences, Latvia

Co-Chair
Catherine Bennetau-Pelissero
University of Bordeaux, France
Session Introduction
Henriqueta Louro
National Institute of Health Dr. Ricardo Jorge (INSA), Portugal
Title: Connecting the cytotoxic and genotoxic effects of multi-walled carbon nanotubes to their physicochemical properties
Time : 15:50-16:20

Biography:
Henriqueta Louro has worked in the analysis of potential mutagenic effects of chemical or physical agents in vitro and in vivo. She is also involved in human biomonitoring studies, namely to investigate the biological effects of chemical exposures (HBM4EU), environmental tobacco smoke and also in populations exposed to background radiation originated from natural sources or resulting from uranium mining debris. Her research work is focused on the impact of xenobiotics from the environmental and occupational settings on the human genome. Her recent work involves nanotoxicology, with participation in European projects (Nanogenotox and NanoReg) as well as in national projects.
Abstract:
Statement of the Problem: The manufactured nanomaterials (NMs) have specific physicochemical properties that confer unique characteristics beneficial for biomedical and industrial applications, but that can also determine nano-bio interactions leading to toxic potential. However, the investigation of the genotoxic properties of NMs has been mostly inconclusive, since divergent results have been reported in the literature. To contribute for the safety assessment of NMs, it is important to try to ascertain the NM characteristic that determines the adverse effects, allowing the synthesis of innovative NMs devoid of toxicity.
Objective & Methodology: The present work explores the correlation between physicochemical properties of benchmark NMs (multi-walled carbon nanotubes, MWCNTs) and their cytotoxic and genotoxic effects in human respiratory cells (A549 and Beas-2B), through the MTT, clonogenic, micronucleus and comet assays.
Conclusion & Significance: After 8-days exposure, the clonogenic assay showed cytotoxic effects in A549 cells for all the tested MWCNTs. Correlation analysis suggested an association between the MWCNT size in cell culture medium and cytotoxicity. No induction of DNA damage was observed after any MWCNT exposure in any cell line by the comet assay, while the micronucleus assay revealed that both NM-401 and NM-402 were genotoxic in A549 cells. NM-401 and NM-402 are the two longest MWCNTs analyzed in this work, suggesting that length may be determinant for genotoxicity. No induction of micronuclei was observed in the Beas-2B cell line. The different effects in both cell lines are explained in view of the size-distribution of MWCNTs in the cell culture medium, rather than cell’s specificities. Therefore, tackling NMs safety issues is a complex and challenging issue. It is mandatory that toxicologists adequately characterize both the primary and secondary physicochemical properties of the test nanomaterials and use several endpoints to allow a correct interpretation of data.
Recent Publications
1. Louro H, Pinhão M, Santos J, Tavares A, Vital N, Silva M J (2016) Evaluation of the cytotoxic and genotoxic effects of benchmark multi-walled carbon nanotubes in relation to their physicochemical properties. Toxicol. Lett. 262: 123-134.
2. Costa J G, Saraiva N, Guerreiro P S, Louro H, Silva M J, Miranda J P, Castro M, Batinic-Haberle I, Fernandes A S, Oliveira N G (2016) Ochratoxin A-induced cytotoxicity, genotoxicity and reactive oxygen species in kidney cells: An integrative approach of complementary endpoints. Food and Chemical Toxicology 87: 65-76.
3. Tavares A, Louro H, Antunes S, Quarré S, Simar S, Temmerman P D, Verleysen E, Mast J, Jensen K A, Norppa H, Nesslany F, Silva M J (2014) Genotoxicity evaluation of nanosized titanium dioxide, synthetic amorphous silica and multi-walled carbon nanotubes in human lymphocytes. Toxicology In Vitro 28: 60-69.
Jayadev Raju
Health Canada, Canada
Title: Toxicological and toxicogenomic responses in male and female F344 rats following 28-days repeated dose sub-acute dietary exposure to 2-monochloro-1,3-propanediol (2-MCPD)
Time : 16:40-17:10

Biography:
Jayadev Raju completed his MSc and PhD in Life Sciences from Jawaharlal Nehru University, New Delhi, India. He received his Post-doctoral training and research expertise in Nutrition and Cancer at the University of Manitoba, Winnipeg Canada; the American Health Foundation Cancer Centre, Valhalla, New York, USA; the German Cancer Research Centre in Heidelberg, Germany; and University of Waterloo, Waterloo, Ontario, Canada. He is currently a Research Scientist in the Federal Food Regulatory Setting (Bureau of Chemical Safety, Health Canada). He provides toxicological research expertise related to carcinogenesis and co-carcinogenesis of foods and food constituents, including those that are classified as additives, contaminants, process-induced compounds and packaging material-migrating chemicals. The main goal of his research is to provide hazard characterization of food-borne chemicals, using both conventional OECD testing guidelines and models of diseases (specifically cancer), for supporting regulatory chemical risk management processes. He is also interested in the biology of precancerous lesions of the colon and their use as a surrogate biomarker in evaluating foods and drugs. He is a recipient of the V E Henderson Award (Society of Toxicology of Canada; 2010) and the International ToxScholar Outreach Award (Society of Toxicology, USA; 2016).
Abstract:
Introduction: Monochloropropanediols (MCPDs) are a class of chemicals that are generated as a result of high temperature processing of vegetable oils and foods that contain such refined oils. The toxicology of 3-MCPD is well-understood and is classified as a ‘possible human carcinogen’ (IARC Group 2B); however, there is insufficient data to characterize the toxicity of 2-MCPD, a related compound.
Objective: This study was conducted to fill a regulatory data gap in identifying the mode of action of dietary 2-MCPD in tissues of F344 rats exposed for 28 days according to the Organization of Economic Cooperation and Development (OECD) test guideline-407 using both apical and toxicogenomic endpoints.
Methodology: Weanling male and female F344 rats (n=10 rats/group/sex) were fed ad libitum AIN-93G diets containing 2-MCPD to provide estimated daily doses of 25, 50, 100 or 200 mg/kg body weight (BW). Rats were killed 28 days (unless found moribund) after exposure and their tissues processed for endpoint analyses.
Findings: Within the first week of exposure, female rats in the 100 and 200 mg/kg BW dose groups of 2-MCPD became moribund and were euthanized. Male rats were spared from exposure to these high doses and these groups were excluded from the study. Non-cancerous lesions with minimal to moderate scores were observed specifically in the kidney and spleen (50 mg/kg BW in males), heart (50 mg/kg BW in females) and thyroid (25 and 50 mg/kg BW in males and 50 mg/kg BW in females). Weights of kidneys in both sexes were significantly higher in the 2-MCPD groups along with higher levels of creatine kinase and lower levels of blood urea nitrogen. Heart weights were significantly higher in the 50 mg/kg BW groups in both sexes. Additionally, we observed significantly lower ALT and AST in males at both 25 and 50 mg/kg BW 2-MCPD, together with lower levels of high-density lipoproteins and cholesterol at 50 mg/kg BW 2-MCPD in both sexes. Genomic data indicated that in treated kidneys, 2-MCPD significantly increased Hmox1 and Ptgs2 genes, both involved intrinsically in inflammation. Several pathways were targeted in the heart as a consequence of 2-MCPD exposure such as angiogenesis, metabolic regulation and cell migration. The liver tissue only showed limited changes in the battery of genes tested.
Conclusion: For 2-MCPD (a) at the lowest tested dose of 25 mg/kg BW, treatment-related changes were notable in the kidneys and thyroid, (b) sex-specific changes in certain biochemical and hematological parameters were apparent, (c) pathological changes were observed in the kidney, heart, and thyroid, and (d) a no-observed-effect level (NOEL) was not reached in this study. Genomic analysis of the three tissues identified differential expression of key genes in the kidney and heart of animals treated with 2-MCPD.
Significance: This detailed sub-acute dietary exposure study provides toxicology and toxicogenomic data to support the hazard characterization of food-borne 2-MCPD for regulatory purposes; however, the lack of a NOEL provides impetus to further study 2-MCPD exposure.
Kobbi Zina
University of Monastir, Tunisia
Title: Comparative repeated toxicity study in rats: Enoxaparin biosimilar product versus reference product
Time : 17:10-17:40

Biography:
Kobbi Zina is a Pharmacist and PhD student, with a broad and acute interest in drug development, regulation and researches. Her long experience in pharmaceutical industry and researches on biosimilars, lead her to be an expert in the field. She has done her Master’s degree in Drug Development and worked on therapeutic equivalence of generic products and BCS classification. She is pursuing her PhD on Biosimilar Evaluation, while working in an industry developing generics and biosimilars. Her research works are mainly focused on preclinical evaluation of a biosimilar (enoxaparin) by performing in vitro comparative studies (physico-chemical and biological) and in vivo comparative studies (toxicity and pharmacodynamics studies).
Abstract:
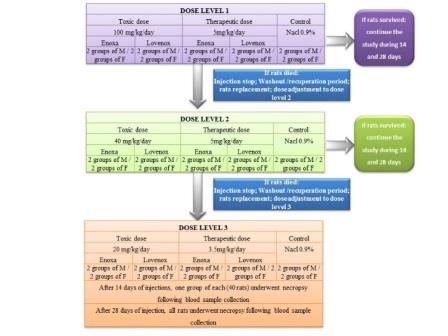
During last decades we observed explosion of biosimilars, which require biosimilarity study including comparative safety evaluation conducted on animals. Enoxaparin is a low molecular weight heparin widely used for the prevention and treatment of thromboembolism and is considered as a biological product. With the development of several enoxaparin biosimilars, real medical concerns about their safety and efficacy were raised. This repeated dose toxicity part of the biosimilarity study consists of preclinical toxicological evaluation of a similar biological version of enoxaparin drug product “Enoxa” manufactured by “Les Laboratories Médis” (Tunisia), compared to the enoxaparin reference drug product “Lovenox” manufactured by Sanofi-Aventis (France). Eighty (80) white Wistar rats were treated with enoxaparin biosimilar, versus the reference product, using subcutaneous therapeutic dose and toxic doses, varying from 3.5 to 100 mg/kg/day. Dose levels were adjusted and ultimately fixed at 3.5 mg/kg/day for a therapeutic dose and 20 mg/kg/day for a toxic dose. A 0.9% sodium chloride solution was used for the control group and the comparative study was conducted over a period of 14 days and 28 days. Animals were observed before and during study, all animal were euthanized at the end of the study design then necropsy, organs sampling and anatomo-histopathology were then performed. Hematology and biochemistry evaluation of relevant parameters was performed on all animals. Comparable effects were observed at all doses and all products with few adverse effects observed at doses 20 mg/kg/day for both enoxaparin biosimilar and reference products. Mortality started at a dose of 40 mg/kg/day and reached 25%, at 100 mg/kg/day for both products. Since results from the similar biological version of enoxaparin drug product “Enoxa” and reference drug product “Lovenox”, have comparable toxicity profile in rats, continuing investigation of biosimilarity on humans to confirm safety and efficacy is suggested.
Recent Publications
1. Kobbi Z, Hfaiedh N, Fenina N (2015) Biosimilar evaluation and structural characterization: A comparison study for enoxaparin. American Journal of PharmTech Research 5(3): 593-607.
Quinteros M A
National Scientific and Technical Research Council (CONICET), Argentina
Title: Biosynthesized silver nanoparticles: Decoding their mechanism of action in S. aureus and E. coli
Time : 17:40-18:10

Biography:
Quinteros M A is pursuing her PhD from National Scientific and Technical Research Council (CONICET). She has done her graduation in Pharmaceutical Chemistry in the year 2009. Her research interests include the study of oxidative stress generated by biosynthesized metallic nanoparticles with antimicrobial activity and its relationship with the bacterial resistance.
Abstract:
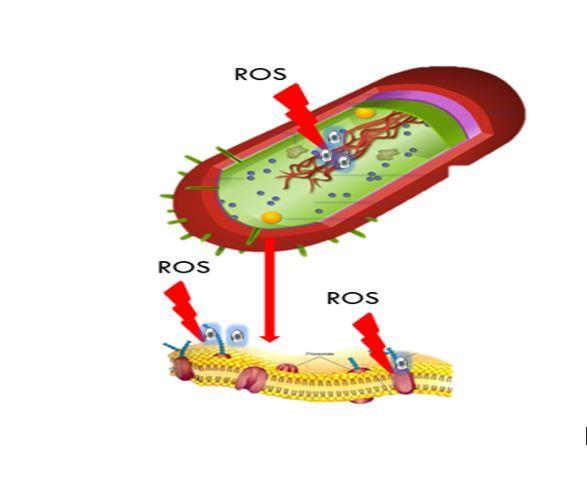
The generation of oxidative stress in bacteria in the presence of silver nanoparticles (AgNPs) is already widely known. If the cell cannot respond to oxidative injury produced by increased species reactive oxygen (ROS), the oxidation of macromolecules such as proteins, lipids and DNA occurs, leading to the death of the bacterium. In previous results, we observed as biosynthesized AgNPs that had antibacterial activity, generated an increase of ROS and RNI in Staphylococcus aureus, Escherichia coli and Pseudomonas aeruginosa, being responsible for their toxicity and bacterial death. According to the above, we set out to delve into the mechanism of action of AgNPs, by determining markers of oxidative stress, such as protein oxidation, lipids, DNA and changes in membrane potential in two reference strains S. aureus ATCC 29213 and E. coli ATCC 25922. We found that the increase in the levels of ROS is associated with the oxidation of different macromolecules important for the normal functioning of the cell, so that oxidative stress would be one of the mechanisms by which the AgNPs would exert their toxicity in these two strains of great clinical relevance. In this way, we are making a great contribution on the toxicity produced by AgNPs.
Recent Publications
1. Quinteros M A, Aiassa Martínez I M, Dalmasso P R, Páez P L (2016) Silver nanoparticles: Biosynthesis using an ATCC reference strain of Pseudomonas aeruginosa and activity as broad spectrum clinical antibacterial agents. Int. J. Biomater. 5971047.
2. Quinteros M A, Cano Aristizábal V, Dalmasso P R, Paraje M G, Páez P L (2016) Oxidative stress generation of silver nanoparticles in three bacterial genera and its relationship with the antimicrobial activity. Toxicol. In Vitro 36:216-23.
- Networking Lunch Session
Location: Sunset 2
- Environmental Toxicology | Drug Toxicology | Occupational Toxicology
Location: Sunset 2
Chair
James Dahlgren
James Dahlgren Medical, USA
Co-Chair
Patrick Talbott
James Dahlgren Medical, USA
Session Introduction
Diana A Stavreva
National Institutes of Health, USA
Title: Fluorescence-based cell assay for high-throughput detection of endocrine-disrupting chemicals (EDCs) in water sources: Principle and applications
Time : 10:35-11:05

Biography:
Abstract:
Endocrine-disrupting chemicals (EDCs) interfere with normal function of the endocrine system and have been associated with metabolic disorders, immune dysfunction, developmental defects, and cancer. Thus, presence of EDCs in the environment and water sources in particular, is a major health concern. Standard detection of EDCs in the environment relies on a laborious analysis of chemical structures using HPLC, MS/GS and related technologies. These methods are costly, time consuming and frequently fail to identify a specific chemical structure as many chemicals are subjected to bio-modifications in the environment. These derivatives cannot be easily identified and are not present in the currently existing libraries. Consequently, their levels are not efficiently monitored or regulated. In addition, it is unclear whether the EDCs detected by chemical methods elicit biological responses in mammalian systems. To overcome these obstacles, we developed a high-throughput assay for biological testing of EDCs using mammalian cell lines that express GFP-tagged nuclear steroid receptor constructs. This assay is based on translocation of fluorescently labeled nuclear receptor construct from the cytoplasm to the nucleus in the presence of EDCs acting on this particular receptor. Using this assay, we detected androgen activity in 35% of the tested water samples, and a previously unrecognized glucocorticoid (GC) activity in 27% of the samples. We also developed a novel call line expressing a GFP-tagged glucocorticoid receptor-thyroid receptor (GFP-GR-TRβ) chimeric construct which could detect known EDCs such as BPA and TBBPA as well as TRβ-interacting contaminants in water samples. We conclude that the wide-spread contamination of water sources with different classes of EDCs is a possible health hazard not only for the aquatic ecosystems, but may also negatively impact the human population. Largely unrestricted human activity with respect to pharmaceuticals and other potential endocrine disruptors is of concern and represents one of the main reasons for these wide-spread contaminations
Branko Petrinec
Institute for Medical Research and Occupational Health, Croatia
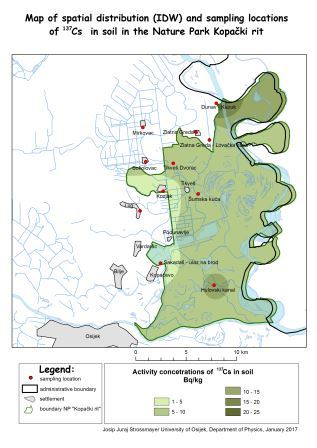
Title: Influence of 137Cs on the wildlife in the KopaÄki Rit nature park, Croatia
Time : 11:05-11:35

Biography:
Branko Petrinec obtained his PhD degree from the Physics department of the University of Zagreb. He is a Research Associate at the Institute for Medical Research and Occupational Health in Zagreb, and an Assistant Professor in the Department of Physics, Josip Juraj Strossmayer University in Osijek. He has published more than 20 papers in reputed journals. He was the President for the Scientific Committee of the 10th Symposium of the Croatian Radiation Protection Association.
Abstract:
We collected samples in accordance with the standard procedures recommended by the International Atomic Energy Association, within an internal project of the Institute for Medical Research and Occupational Health and in collaboration with the Physics department of the University of Osijek. The activity concentration of 137Cs was determined using a high-purity germanium gamma-ray spectrometry system (FWHM of 2.24 keV and relative efficiency of 74.2%, all at 1.33 MeV 60Co). The measured activity concentrations in soil from 11 locations range from 1.81±0.16 Bq/kg (Kozjak) to 10.65±0.26 Bq/kg (Hulovski channel), averaging to of 7.20±5.57 Bq/kg which is comparable to other parts of Croatia. Data sets consisting of measured activity concentrations of 137Cs in soil and water samples within the boundaries of the Nature Park Kopacki Rit were used for the risk assessment for the wildlife and dose rates for freshwater and terrestrial organisms were estimated. For the freshwater ecosystem in KopaÄki Rit, the highest risk quotient (RQ) values were obtained for “pelagic fish” 0.038 and “amphibian” 0.037, respectively. Appropriate dose rates were below the screening level of 10 μGy h-1: amphibian 0.37 μGy h-1, bird 0.27 μGy h-1, insect larvae 0.12 μGy h-1, mammal 0.29 μGy h-1, pelagic fish 0.38 μGy h-1 and vascular plant 0.022 μGy h-1. Also, for terrestrial ecosystems, all RQs are far below 1 (the highest values are 0.0013 for “lichen”, 0.0019 for “small mammal” and 0.0027 for “large mammal”) and the dose rates to species studied were clearly below the screening level of 10 μGy h-1 (one-thousandth of this value: 0.0128 μGy h-1 for the lichen, 0.0196 μGy h-1 and 0.0277 μGy h-1 for small and large mammals, respectively) indicating no significant impact of the 137Cs fallout on all of the investigated species.
Recent Publications
1. PaviÄić-Hamer D, Barišić D, Šimunac B, Petrinec B, Štrok M (2016) 137Cs distribution in the Northern Adriatic Sea. Journal of Radioanalytical and Nuclear Chemistry 309: 989-998.
2. Franić Z, Šega K, Petrinec B, Marović G (2009) Long-term investigations of post-Chernobyl radiocaesium in fallout and air in North Croatia. Environmental Monitoring and Assessment 148: 315-323
3. Petrinec B, Štrok M, Franić Z, Smodiš B, PaviÄić-Hamer D (2013) Radionuclides in Adriatic Sea and related dose rate assessment for marine biota. Radiation protection dosimetry 154(3): 320-330.
4. Šoštarić M, Petrinec B, Babić D (2013) 137Cs in soil and fallout around Zagreb (Croatia) at the time of the Fukushima accident. Archives of Industrial Hygiene and Toxicology 64(4): 561-565.
5. Franić Z, Petrinec B, Marović G, Franić Z (2007) Radiocaesium activity concentrations in potatoes in Croatia after the Chernobyl accident and dose assessment. Journal of Environmental Science and Health - Part B: Pesticides, Food Contaminants, and Agricultural Wastes 42(2): 211-217.
Gyuseong Cho
KAIST, South Korea
Title: A new radiation scale for the public: RAIN
Time : 11:35-12:05

Biography:
Gyuseong Cho is a Professor of the Department of Nuclear and Quantum Engineering at KAIST (Korea Advanced Institute of Science and Technology) since 1994. He has expertise in design and evaluation of radiation detectors and radiation imaging systems for medical and industrial applications. Particularly he contributed to the development of digital radiography detectors based on the amorphous silicon as well as the brain PET systems based on the silicon photomultiplier. Currently he is the President of the Korean Society of Radiation Industry.
Abstract:
We are proposing a new radiation scale, RAIN (radiation index) for the general public who often suffers from misunderstanding and unnecessary fear of the radiation and radiation release accidents. RAIN is defined in dimensionless such as the seismic scale. RAIN is defined to scale the individual accumulated radiation dose originating from an arbitrarily-defined particular event such as a single medical CT examination, an intake of contaminated foods or an exposure due to a certain incident for a certain period of time. The practical range of RAIN value varies from zero to 10. To maximize the simplicity for the general public, the numerical value of RAIN shall retain no more than a single significant digit after decimal point. The reference dose is selected as 10 µSv in a year in this paper, based on the exemption and clearance levels which were suggested by IAEA. Some example values of RAIN are 0.8 for a typical chest X-ray examination (0.07 mSv), 2.9 for a CT examination (7.4 mSv), 3.7 for the maximum annual effective dose limit of a radiation worker (50 mSv), 5.6 for the lethal dose LD50/60 (4000 mSv) etc. As a quick aid for the general public, the last column of Table 1 provides the three zones of RAIN values (Green, Yellow, and Red zones) which are characterized by the degree of severity of radiation exposure. We anticipate that this new definition of RAIN may serve the public and the experts to converse more correctly, easily and fruitfully.
Recent Publications
1.Cho G, Kim JH, Park TS, Cho K (2017) Proposing a Simple Radiation Scale for the Public: RAIN. NET (to be published in April, 2017)
2. BIPM, the International System of Units, Bureau International des poids et measure, 8th Ed., 2006, http://www.bipm.org/en/publications/si-brochure/section2-2.html
3.IAEA General Safety Requirements, GSR Part 3 BSS, 2014
Danju Zhang
Sichuan Agricultural University, China
Title: Allelopathy of Eucalyptus grandis
Time : 12:05-12:35

Biography:
Danju Zhang has her expertise in silviculture, forest ecology, chemical ecology. She along with her team has focused on the short rotation Eucalyptus plantations for over 25 years. She identified the volatile organic compounds and water-soluble phenol allelochemicals and evaluate their physio-ecological effects on plant and soil biota.
Abstract:
Allelopathy has been identified as an underlying mechanism of detrimental environmental impacts within commercial plantations. Eucalyptus spp. is known to generate huge amounts of volatile organic compounds (VOCs) that can function as phytotoxins and thus inhibit other plants. In the present study, biochemical markers, including activities of acetylcholinesterase (AChE) and oxidative stress enzymes, such as superoxide dismutase (SOD) and glutathione S-transferase (GST), were assayed to assess changes in Eisenia fetida at the physiological level induced by different doses of VOCs as part of an acute toxicity test over 7 and 14-day exposures. In addition, the toxicities of VOCs were investigated using a soil avoidance test and comet assay. The results revealed that E. fetida exhibited significant avoidance behavior towards the highest concentrations of undecane, decane, 2,4-dimethyl heptane, and 2,2,4,6,6-pentametyl heptane. The tail DNA percentages were significantly increased for all experimental treatments relative to control. However, under the treatments of VOCs, Olive tail moment content and comet tail length also display an obvious increase compared to control, except for that of octane, undecane and decane treatments. As VOC concentrations and durations increased in the soil, activities of AChE, SOD, and GST were either stimulated or inhibited. Among the VOCs, decane, 2,4-dimethyl heptane, 2,2,4,6,6-pentamethyl heptane, and 2,4-di tert buyl phenol exerted stronger effects on enzymatic activities. In summary, VOCs in rhizosphere soils of E. grandis might exert a toxic impact on E. fetida, among which 2,4-dimethyl heptane, 2,2,4,6,6-pentamethyl heptane, and 2,4-di tert buyl phenol have the strongest effects. It was found that 4 and 8 year might be the important turning points for the dynamics of the allelochemicals. The effects of water-soluble allelochemicals were stronger than that of VOCs. At present, selection of the main effective phenol compounds through UPLC-MS and their physioecological effects are in progress.
Recent Publications
1.Tang Z Q, Zhang J, Yu J L, Wang C Z, Zhang D J (2017) Allelopathic effects of volatile organic compounds from Eucalyptus grandis rhizosphere soil on assessed using avoidance bioassays, enzyme Eisenia fetida activity and comet assays. Chemosphere 173(4): 307-317.
2.Wang C Z, Zhang D J, Zhang J, Ji T W, Tang Z Q, Zhao Y Y (2015) Allelopathic effects of volatile compounds from Eucalyptus grandis on Vigna radiata, Raphanus sativus and Lactuca sativa. Allelopathy Journal 36(2): 133-325.
3.Zhang D J, Zhang J, Yang W Q, Wu F Z, Huang Y M (2014) Plant and soil seed bank diversity across a range of Eucalyptus grandis plantations afforested on arable lands. Plant and Soil 376: 307-325.
- Special Session
Location: Sunset 2
Session Introduction
Vijay Jagdale
Sai Life Sciences Ltd., India
Title: Strategy for the early drug discovery assays and toxicology screening
Biography:
Vijay Jagdale is Veterinarian by profession and has expertise in Preclinical Toxicology as a Toxicologist as well as Pathologist working with various CRO’s and discovery/generic pharmaceutical companies. He has contribution in building strategic discovery platform for preclinical assays to facilitate smooth pathway for the lead candidates. He has expertise in the area of preclinical research in drug discovery and development in CROs with adequate exposure to preclinical toxicology, toxico-pathology, DMPK and laboratory animal facility set up, management and operation. Complete understanding of preclinical GLP toxicology with multiple types of therapeutics areas adhering to the various national and international regulatory guidelines. He also has experience in establishment, operation and management of national and international (AAALAC/OLAW) accredited rodent facilities with preclinical toxicology laboratory.
Abstract:
Developing a new drug from original idea to the launch of a finished product is a complex process where strategic planning plays crucial role to reduce usual time (12–15 years) as well as cost ( 1 billion $) for all the processes. The pharmaceutical industry has been evolving worldwide in the recent years, which made numerous CROs and biotech companies conducting such programs through services in various countries. The proper selection and applications of correct models, as well as appropriate data interpretation, are critically important in decision making and successful advancement of drug candidates. The successful discovery program includes strategic execution and read outs from initial assays like physicochemical characterization (kinetic solubility, thermodynamic solubility, pH dependent solubility and lipophilicity), absorption (PAMPA, MDCK permeability, Caca-2 permeability, MDCK-MDR1 permeability), metabolism (liver microsomes, hepatocytes, S9 fraction, reaction phenotyping, CYP inhibition and time dependent inhibition) and other preliminary assays (PPB, brain/tissue binding, blood to plasma ration, plasma stability, SGF/SIF stability, buffer stability, etc.) to prioritize leads for the further in-vivo assays. Pharmacokinetics assays needs appropriate execution to rank order compounds based on promising clearance (CL), bioavailability (F%), exposure (AUC), half-life (t1/2), and distribution volume (L). Selective in-vivo PK studies are valuable to confirm whether the applied in vitro assays (in vitro metabolism and absorption) can serve as good predictive models for in vivo PK in terms of plasma clearance and bioavailability. The early application of preclinical safety assessment-both new molecular technologies as well as more established approaches such as standard repeat-dose rodent toxicology studies can identify predictable safety issues earlier in the testing paradigm. These earlier identification of dose-limiting toxicities will provide chemists and toxicologists the opportunity to characterize the dose-limiting toxicities, determine structure–toxicity relationships and minimize or circumvent adverse safety liabilities. Early stages includes nonclinical safety studies on candidate drugs to assess general toxicology (through in vivo experiments), safety pharmacology (effects on major organ systems) and basic genetic toxicity tests during early discovery phase in non-GLP conditions. Because of this, there is a strong need for personnel involved with toxicology and pharmacology studies need to understand the varied tools and approaches to perform early drug discovery safety analysis.
- Sessions on: Environmental Toxicology | Drug Toxicology | Occupational Toxicology
Location: Sunset 2
Chair
James Dahlgren
James Dahlgren Medical, USA
Co-Chair
Patrick Talbott
James Dahlgren Medical, USA
Session Introduction
Lucija Peterlin MaÅ¡iÄ
University of Ljubljana, Slovenia
Title: Bisphenol A analogs and new brominated flame retardants: Do their metabolites have endocrine activity?
Time : 14:20-14:50

Biography:
Lucija Peterlin MašiÄ is an Associate Professor for Medicinal Chemistry and an Assistant Professor for Toxicological Chemistry. She has expertise in Medicinal Chemistry and Toxicology. She has studied the influence of metabolism on endocrine activities of hormone disrupting chemicals such as analogs of bisphenol A and novel brominated flame retardants with different in vitro assays. Her research interests are also in vitro studies of drug metabolism, testing compounds acting on nuclear receptors (estrogen, thyroid, androgen, glucocorticoid, PXR, FXR and PPARs), metabolism of xenobiotics and activity studies of their metabolites, bimolecular mechanism studies of toxicity, structure and ligand based drug design, etc.
Abstract:
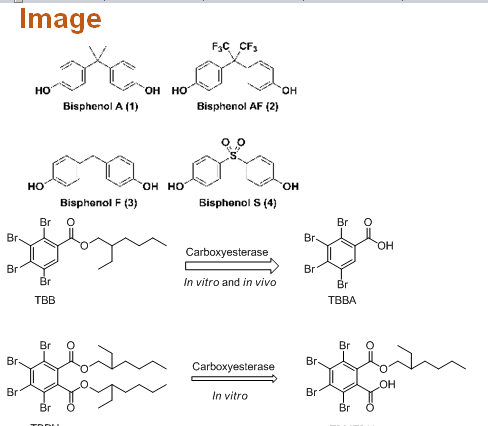
Statement of the Problem: Structural analogs of bisphenol A (BPF, BPAF, BPS) are commonly used as its alternatives in industrial and commercial applications. Nevertheless, the question arises whether the use of other bisphenols is justified as replacements for bisphenol A in mass production of plastic materials. Knowledge about the metabolic pathways and enzymes involved in metabolic bio-transformations is essential for understanding and predicting mechanisms of toxicity. The activities on different nuclear receptors of the new brominated flame retardants 2-ethylhexyl 2,3,4,5-tetrabromobenzoate (TBB) and bis(2-ethylhexyl) 2,3,4,5-tetrabromophthalate (TBPH), and their main carboxylic acid metabolites 2,3,4,5-tetrabromobenzoic acid (TBBA) and mono(2-ethylhexyl) tetrabromophthalate (TBMEPH) were also investigated.
Methodology & Theoretical Orientation: The bisphenols metabolism using pooled liver and intestine microsomes, as well as recombinant human cytochromes (CYP1A2, CYP2C8, CYP2C9, CYP2C19, CYP2E1, and CYP3A4) was tested using LC-MS/MS. For nuclear receptor testing in vitro systems were used to evaluate the estrogenic, androgenic, glucocorticoid, thyroid, PXR and PPARs activities of parent chemicals and their metabolites.
Conclusion & Significance: Once enter the body; the bisphenols are subjected to both oxidative metabolism and mainly conjugation. However, conjugation, which is mainly with glucuronic acid, is the predominant metabolic pathway for bisphenols and this is therefore considered to be an important mechanism for bisphenols detoxification. However, comprehensive testing of BPAF-glucuronide on all nuclear receptors has been performed and will be presented. On the other hand, it has been detected that metabolites of bisphenols and flame retardants have enhanced endocrine activities. For example, the estrogenic activities of MBP and hydroxycumyl alcohol are significantly higher than that of BPA. Even the formation of small amounts of those metabolites can significantly affect estrogenic activity. This was observed also for the novel brominated flame retardants TBB and TBPH, which showed weak or no activity on estrogen and androgen receptors, while their metabolites showed significant anti-estrogenic and anti-androgenic effects. Although conjugation is an extremely important detoxification pathway for bisphenols, it cannot completely eliminate their toxic effects in the body. Environmental estrogens can elicit their responses not only through binding to ERs, but also through numerous alternative pathways, and very low concentrations are needed for measurable effects. Despite their rapid glucuronidation, small amounts of bisphenols remain in their free form. As these can be further metabolized to their biologically active metabolites, these represent the risk for human health.
Recent Publications
1. Gramec D, Schmidt J, Fic A, KlopÄiÄ I, Trontelj J, Sollner Dolenc M, Finel M, Peterlin MašiÄ L (2016) Influence of metabolism on endocrine activities of bisphenol S. Chemosphere 157: 152-159.
2. KlopÄiÄ I, Gramec D, Peterlin MašiÄ L, Sollner Dolenc M (2016) Comparison of in vitro hormone activities of novel flame retardants TBB, TBPH and their metabolites TBBA and TBMEPH using reporter gene assays. Chemosphere 160: 244-251.
3. Gramec D, TomašiÄ T, Carino A, Distrutti E, Fiorucci S, Peterlin MašiÄ L (2016) New brominated flame retardants and their metabolites as activators of the pregnane X receptor. Toxicology Letters 259: 116-123.
4. Fic A, JurkoviÄ Mlakar S, Juvan P, Mlakar V, Marc J, Sollner Dolenc M, Broberk K, Peterlin MašiÄ L (2015) Genome-wide gene expression profiling of low-dose, long-term exposure of human osteosarcoma cells to bisphenol A and its analogs bisphenols AF and S. Toxicology In Vitro 29: 1060-1069.
5. Fic A, Žegura B, Gramec D, Peterlin MašiÄ, L (2014) Estrogenic and androgenic activities of TBBA and TBMEPH, metabolites of novel brominated flame retardants, and selected bisphenols, using the XenoScreen XL YES/YAS assay. Chemosphere 112: 362-369.
Yassine Ait Bali
Cadi Ayyad University, Morocco
Title: Neurobehavioral and cognitive changes in mice offspring following prenatal exposure to paraquat
Time : 14:50-15:20

Biography:
Yassine Ait Bali is a Research Neurocientist at the Pharmacology, Neurobiology and Behavior Laboratory. His research interests have focused on the Neurotoxicology field. He is concentrating mainly on the relationship between pesticides side effects and the onset of brain diseases especially following developmental exposures covering all the aspects of neurological disease at different levels: Behavioral, morphological, physiological and molecular.
Abstract:
Statement of the Problem: The herbicide paraquat is a wide used product. Its role in the pathogenesis of some brain disorders has become intensely debated and gained increasing interest in recent years. The aim of our study is to investigate the developmental and neurobehavioral effects of prenatal exposure to this product in mice.
Material & Methodology: In the present work, we investigated the acute and developmental toxicity of PQ, from the 1st or 6th day of mating and throughout the gestation period. We have examined several parameters, including toxicity indices, reproductive performance and sensorimotor development, as well as anxiety and cognitive performance of the offspring.
Findings: Our results showed that exposure to 20 mg/kg of Paraquat during the first days of pregnancy completely prevent pregnancy in treated mice. Ingestion of a tolerable dose from the 6th day of pregnancy caused an alteration in fertility and reproductive parameters and a decrease in litter size. In offspring, paraquat is responsible for a variety of behavioral disorders, manifested as an overall delay of innate reflexes and a deficit in motor development. All exposed animals showed a decrease in the level of locomotor activity, increased levels of anxiety and pronounced cognitive impairment in adulthood. Immunohistochemical studies have shown a decrease in the number of TH-immunoreactive neurons in the substantia nigra and intense glial proliferation in the hippocampus (using GFAP) in treated animals.
Conclusion & Significance: These results demonstrated that paraquat led to the onset of many behavioral changes that stem from the impairment of neuronal developmental processes in prenatally exposed mice.
Jacqueline Chuah
Institute of Bioengineering and Nanotechnology, Singapore
Title: Human stem cell-derived renal cells and in vitro platforms for nephrotoxicity prediction
Time : 15:20-15:50

Biography:
Jacqueline Chuah received her B.Sc (Hons) in Biological Sciences from the Nanyang Technological University, Singapore. In 2013 she joined Dr. Daniele Zink’s team at the Institute of Bioengineering and Nanotechnology of the Agency for Science, Technology and Research, Singapore. Dr. Zink’s lab develops organ-specific predictive screening technologies for in vitro toxicology/ nanotoxicology and conducts stem cell research with an emphasis on applications in predictive toxicology. Jacqueline’s work is focused on developing stem cell-based assays to predict kidney toxicity.
Abstract:
There is an increasing demand for alternative methods for the evaluation of compound toxicity in humans. Accepted alternative methods for the prediction of toxicities for human internal organs, such as liver and kidney, are not available. We have developed the first pre-validated platforms for the prediction of compound-induced nephrotoxicity in humans (Chuah and Zink, 2017; Kandasamy et al., 2015; Li et al., 2014; Li et al., 2013; Su et al., 2014; Su et al., 2016). These methods include the first high-throughput platform for the accurate prediction of nephrotoxicity (Su et al., 2016), as well as the first and only predictive stem cell-based methods (Li et al., 2014; Kandasamy et al., 2015). Our stem cell-based methods employ a rapid and robust one-step protocol for the differentiation of human induced pluripotent stem cells (iPSC) into renal proximal tubular cell (PTC)-like cells. PTC are a major target for compound-induced toxicity in the kidney due to their roles in compound transport and metabolism. iPSC-derived PTC-like cells have a purity of >90% after only 8 days of differentiation (Kandasamy et al., 2015). They can be directly applied for compound screening, as no cell purification is required. A method for the prediction of nephrotoxicity has been developed by combining use of iPSC-derived PTC-like cells with machine learning methods. This method has been pre-validated with 30 compounds and has a test balanced accuracy of 87% (Kandasamy et al. 2015). Furthermore, the underlying mechanisms of drug-induced cellular injury could also be correctly identified (Kandasamy et al., 2015). PTC-like cells are also suitable for applications in our high-throughput platform, which combines high-content imaging of renal cells with automated phenotypic profiling and machine learning methods (Su et al., 2016). This automated platform has been pre-validated with 44 compounds and has a test balanced accuracy ranging between 82% - 89%, depending on the human renal cell type used (Su et al., 2016). Based on these technologies we are currently developing a portfolio of platforms for the prediction of various organ-specific toxicities. In addition, we have established an organ-on-chip platform for repeated dose testing. This robust platform is based on simple and disposable chips and may be suitable for predicting quantitative parameters of the human dose response, such as No Observed Adverse Effect Levels.
Recent Publications
1. Chuah J K C, Zink D (2017) Stem cell-derived kidney cells and organoids: Recent breakthroughs and emerging applications. Biotechnology Advances 35: 150-167.
2. Kandasamy K, Chuah J K C, Su R, Huang P, Eng K G, Xiong S, Li Y, Chia C S, Loo L H, Zink D (2015) Prediction of drug-induced nephrotoxicity and injury mechanisms with human induced pluripotent stem cell-derived cells and machine learning methods. Scientific Reports 5: 12337.
3. Li Y, Kandasamy K, Chuah J K C, Lam Y N, Toh W S, Oo Z Y, Zink D (2014). Identification of nephrotoxic compounds with embryonic stem cell-derived human renal proximal tubular-like cells. Molecular Pharmaceutics 11: 1982-1990.
4. Su, R., Li, Y., Zink, D., and Loo, L.-H. (2014). Supervised prediction of drug-induced nephrotoxicity based on interleukin-6 and -8 expression levels. BMC Bioinformatics 15, S16.
5. Su R, Xiong S, Zink D, Loo L H (2016) High-throughput imaging-based nephrotoxicity prediction for xenobiotics with diverse chemical structures. Archives of Toxicology 90: 2793-2808.
- Special Session II
Location: Sunset 2
Session Introduction
Vijay Jagdale
Sai Life Sciences Ltd. India
Title: Discovery preclinical screenings using animals with ethical considerations adhering to 3R concept
Biography:
Vijay Jagdale is Veterinarian by profession and has expertise in Preclinical Toxicology as a Toxicologist as well as Pathologist working with various CRO’s and discovery/generic pharmaceutical companies. He has contribution in building strategic discovery platform for preclinical assays to facilitate smooth pathway for the lead candidates. He has expertise in the area of preclinical research in drug discovery and development in CROs with adequate exposure to preclinical toxicology, toxico-pathology, DMPK and laboratory animal facility set up, management and operation. Complete understanding of preclinical GLP toxicology with multiple types of therapeutics areas adhering to the various national and international regulatory guidelines. He also has experience in establishment, operation and management of national and international (AAALAC/OLAW) accredited rodent facilities with preclinical toxicology laboratory.
Abstract:
Historically, animals have been used in a wide range of scientific research activities that have provided many benefits to society, particularly in relation to the advancement of scientific knowledge, human and veterinary medicine, and the safety of chemical products. These principles seem to unify concerns for better science with causing less pain and distress to the experimental animals. All research with the Animal has the potential to cause pain, suffering, distress or lasting harm to the animals used. Most animals are killed at the end of experiments. Practical advances in the scientific methods can reduce areas of conflict. For this reason, the importance of the three R’s (refinement, reduction and replacement), and especially of the need to find replacements, cannot be overstated. The CCAC's ethic of animal experimentation is based on Smyth's definition of alternatives, i.e. replacement, reduction and refinement; replacement alternative methods which avoid or replace the use of animals. This includes both absolute replacements and relative replacements. Reduction alternatives refers to any strategy that results in fewer animals being used to obtain sufficient and precise data to answer the research question, without compromising animal welfare. Refinement alternatives refer to the modification of experimental procedures to minimize pain and distress. The 3Rs are rich in ambiguities and any implementation requires resolving the dilemma that promoting one R will sometimes directly or indirectly conflict with promoting another. The role of national and international accreditations have considerable impact on maintaining standard animal care and use program while designing animal experiments during discovery phase. IACUC/IAEC authority has responsibility to monitor the animal program as well as approvals of experimentation with ethical considerations. The ideas behind the 3Rs are so intuitively compelling that it is tempting to believe that full implementation is merely a matter of time, and once the 3Rs are widely implemented, the public will fully support any continued laboratory animal use that is deemed necessary in the biomedical research.
